"Methamphetamine - The only feature that greatly distinguishes this congener of amphetamine from amphetamine itself is the exceedingly large number of trade names under which this material is sold: to name some, Amphedroxyn, Apamine, Deofed, Desamine, Desoxedrine, Desoxo-5, Desoxyephedrine, Desoxyn, Desvphed, Detrex, Dexoval, Dexstim, D-O-E, Doxyfed Drinalfa, Efroxine, Lanazine, Methamphin, Methedrine, Methoxyn, Miller-Drine, Xorodin, Oxyfed, Oxydess, Premodrin, Normadrine, Xorodin, Semoxydrine, Stimdex, and Syndrox. There is no evidence that its action differs from that of amphetamine in any way except that the action on the cardiovascular system is somewhat less intense and the action on the central nervous system is somewhat more intense. This drug is probably abused more than any other of the group."
-- Diet pill (amphetamines) traffic, abuse and regulation: hearings before the Subcommittee to Investigate Juvenile Delinquency of the Committee on the Judiciary, United States Senate, 92nd Congress, February 1972.
What is most troubling to me about the Diagnostic and Statistical Manual is that is believed to be a resource based on scientific data by the uninformed. Subjective observation and opinion is considered to be the most unreliable type of evidence in science; yet it was heavily relied upon and even used as a substitute for empirical data by the APA when the DSM was first developed and in all subsequent revisions. Every other field of scientific study treats subjective data as if it is unreliable, and it's use is limited to supporting available empirical data. I'm not claiming there is no science behind the DSM; what I am pointing out is the APA relies on consensus which is a quasi-democratic political process; not a scientific one. Consensus is relied on in the absence of empirical data, not to support empirical data. Even educated opinions can not be the basis of ethical diagnostic criteria or ethical medical treatment algorithms and protocols. A medical degree and medicial license do not make professional opinions unbiased, or scientifically valid. Even a a consensus of educated subjective observations can't be empicical evidence that validates diagnostic criteria or treatment recommendations; consensus is a quasi-democratic political process, not a scientific method. Consensus also can't validate the specious claim that psychiatric diagnoses are diseases, or caused by neurobiological diseases or chemical imbalances that require psychiatric treatment. If a patient refuses to give consent for treatment, the APA endorses compelling the person to be forced to have psychiatric treament under color of law by petitioning for court orders requiring involuntary psychiatric treatment. Perhaps it is group delusion that causes members of the APA to believe consensus is a sufficient substitute for empirical data. It is not.
I started writing Involuntary Transformation September 4th, 2010 because of how the political process of psychiatric diagnosis and treatment has been carried out in my son's case. In the summer of 2010 for the first time in over five years he had a crisis due to attempting to process his pain-filled childhood memories. He said he wanted to be hospitalized, and so his elder brother and I accompanied him to the Crisis Center. Nancy Sherman, the Designated Mental Health Professional committed two felonies to have my son Detained under Washington State's Involuntary Commitment laws; committing perjury and forgery to "support" her Emergent Order to Detain. Jeffrey Jennings, a psychiatrist, committed perjury (providing only the same false information Ms. Sherman provided in her original order, not first-hand information as the Law requires--he had no 'first-hand information). Jennings 'treated' my son without regard for the Hippocratic Oath, or Medical Ethics; never speaking to anyone who knew Isaac the entire time he was his 'attending' physician. The Deputy Prosecutor, Dan Polage, and the attorney from Yakima County's Office of Assigned Counsel, Jennifer Lesmez, both failed to conform to the Ethical Guidelines of the Legal profession--as both of these Officers of the Court were aware that perjured testimony was being used. The Crisis Center is run by Central Washington Comprehensive Mental Health and this medicaid provider shredded all the original Superior Court Documents in violation of Washington State Law. The CEO Rick Weaver, told me, "We do it all the time."
I'm not a doctor, or an attorney; I am a mother. I am outraged at the utter lawlessness with which psychiatry is practiced. The events of a year ago were not the first time I have witnessed my son being seriously harmed by unethical "professionals;" and it may not be the last; but as God is my witness, I will not be silent about crimes victimizing my son. It is MY little boy who is now a grown man struggling with the reality of being repeatedly traumatized by unethical mental health professionals that "were supposed to be helping me" but "had no compassion for me, mom" he stated shortly before his crisis in 2010. He asked me in an agony-filled voice, "How could they take so much from me, mom?" I'm at a total loss to explain the callous disregard shown to my son.
My memories of the past almost twenty years are filled with instances--too many to count, of my son speaking of his pain-filled experiences. I have many times felt crushed by the knowledge I was not allowed to protect my own son. Worse, I was prevented from fulfilling his agonized pleas for rescue. These horrific experiences left me utterly devastated. I don't wish to forget my own experiences of bearing witness to my son's torture; I know some horrors must never be forgotten...
"IF we want to understand violence as a whole, we cannot leave any of its major manifestations in a fog of half-knowledge. But this is exactly what has happened with an unprecedented occurrence of mass violence, the deliberate killing of large numbers of mental patients, for which psychiatrists were directly responsible. To both the general public and the psychiatric profession, the details and the background are still imperfectly known. This is not only a chapter in the history of violence; it is also a chapter in the history of psychiatry. Silence does not wipe it out, minimizing it does not expunge it. It must be faced. We must try to understand and resolve it." FREDRIC WERTHAM, M.D A Sign for Cain An Exploration of Human Violence 1966
The other reason I write two blogs is my belief that if I were silent about what was done to my son and my family, I would be complicit in what are crimes against humanity. I will never be complicit. I witnessed Isaac being horribly abused and medical neglected by psychiatric researchers who acted under color of law when they tortured and disabled my son Isaac in a locked research facility.
From the British Psychological Association's Response to the American Psychiatric Association:
DSM-5 Development:
General Comments
"The Society is concerned that clients and the general public are negatively affected by the continued and continuous medicalisation of their natural and normal responses to their experiences; responses which undoubtedly have distressing consequences which demand helping responses, but which do not reflect illnesses so much as normal individual variation. We therefore do welcome the proposal to include a profile of rating the severity of different symptoms over the preceding month. This is attractive, not only because it focuses on specific problems (see below), but because it introduces the concept of variability more fully into the system. That said, we have more concerns than plaudits.
The putative diagnoses presented in DSM-V are clearly based largely on social norms, with 'symptoms' that all rely on subjective judgements, with little confirmatory physical 'signs' or evidence of biological causation. The criteria are not value-free, but rather reflect current normative social expectations. Many researchers have pointed out that psychiatric diagnoses are plagued by problems of reliability, validity, prognostic value, and co-morbidity.
Diagnostic categories do not predict response to medication or other interventions whereas more specific formulations or symptom clusters might (Moncrieff, 2007). Finally, disorders categorised as ‘not otherwise specified’ are huge (running at 30% of all personality disorder diagnoses for example).
Personality disorder and psychoses are particularly troublesome as they are not adequately normed on the general population, where community surveys regularly report much higher prevalence and incidence than would be expected. This problem – as well as threatening the validity of the approach – has significant implications. If community samples show high levels of ‘prevalence’, social factors are minimised, and the continuum with normality is ignored. Then many of the people who describe normal forms of distress
like feeling bereaved after three months, or traumatised by military conflict for more than a month, will meet diagnostic criteria." read the entire response: The British Psychological Association on DSM 5
this is one of Isaac's favorite songs
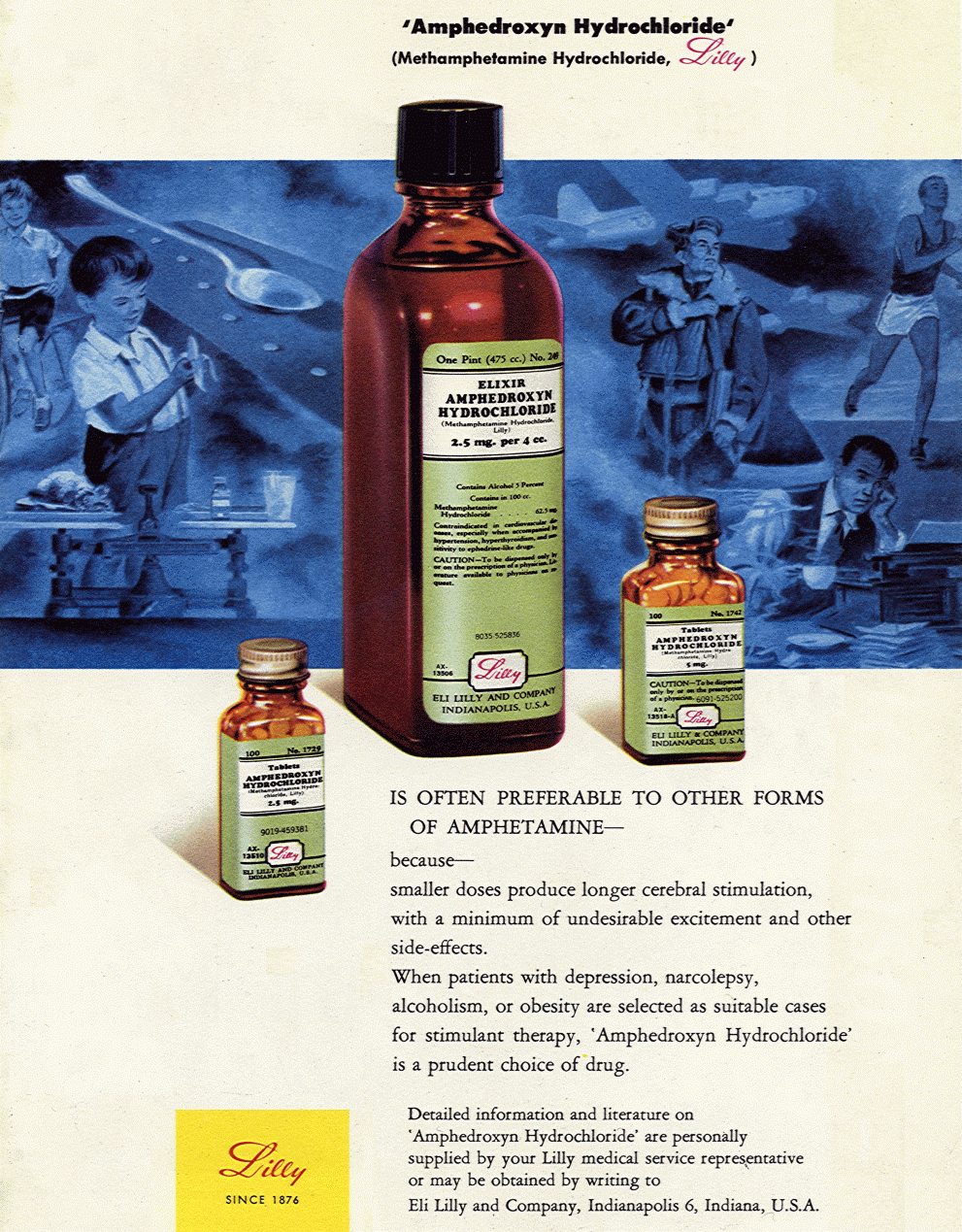
Eli Lilly Amphedroxyn (methamphetamine) advertisement, 1951.
New York State Journal of Medicine, Vol. 51, No. 1.
IS OFTEN PREFERABLE TO OTHER FORMS OF AMPHETAMINE ~ because ~ smaller doses produce longer cerebral stimulation, with a minimum of undesirable excitement and other side-effects.
When patients with depression, narcolepsy, alcoholism, or obesity are selected as suitable cases for stimulant therapy, Amphedroxyn Hydrochloride is a prudent choice of drug.
Contraindicated in cardiovascular diseases, especially when accompanied by hypertension, hyperthyroidism, and sensitivity to ephedrine-like drugs.
CAUTION ~ To be dispensed only by or on the prescription of a physician. Literature available to physicians on request.
Detailed literature on Amphedroxyn Hydrochloride are personally supplied by your Lilly medical service representative or may be obtained by writing to Eli Lilly and Company, Indianapolis 6, Indiana, U.S.A.
LILLY Since 1876
* * * vintage drug ad via Bonker's Institute of Nearly Genuine Research
first posted 8-5-2011 revised 4-17-12, 4-3-2014
first posted 8-5-2011 revised 4-17-12, 4-3-2014







